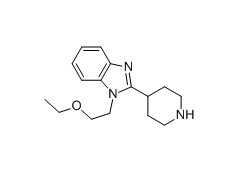
Name: bilastine intermediate 1 1-(2-ethoxyethyl)-2-piperidin-4-ylbenzimidazole
Appearance: white powder
Purity: 99%
CAS Number: 110963-63-8
Molecular formula: C16H23N3O
Molecular weight: 273.37300
Exact quality: 273.18400
PSA: 39.08000
LogP: 2.86860
Bilastine (trade name Bilaxten) is a second generation antihistamine drug for the treatment of allergic rhinoconjunctivitis and urticaria (hives).
It exerts its effect as a selective histamine H1 receptor antagonist, and has a effectiveness similar to cetirizine, fexofenadine and desloratadine. It was developed in Spain by FAES Farma.
Bilastine is approved in the European unio for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria, but it is not approved by the U.S. Food and Drug Administration for any use in the United States. Bilastine meets the current European Academy of Allergy and Clinical Immunology (EAACI) and Allergic Rhinitis and its Impact of Asthma (ARIA) criteria for medication used in the treatment of allergic rhinitis.
Bilastine has been effective in the treatment of ocular symptoms and diseases of allergies, including rhinoconjuctivitis. Additionally, bilastine has been shown to improve quality of life, and all nasal and ocular symptoms related to allergic rhinitis.