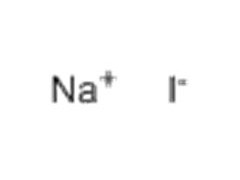Sodium Iodide is a Sodium salt of Hydriodic Acid [HI], available in white crystalline powder form, and has Molecular Formula NaI. Sodium Iodide is highly soluble in water. Sodium Iodide used generally as a reactant for conversion of alkyl chloride, or alkyl bromide into alkyl iodide in presence of Acetone [Finkelstein Reaction]. Due to its hygroscopic nature, it absorbs moisture rapidly from atmosphere. It is produced by treating Iodine with Sodium Hydroxide.